Name: n-butane (or just butane)
Molecular formula: C4H10
Condensed structure: CH3CH2CH2CH3
In stunning 3-D:
 Yes, I just figured out that I could render butane three-dimensionally with my nifty software. Anyway...
Yes, I just figured out that I could render butane three-dimensionally with my nifty software. Anyway...
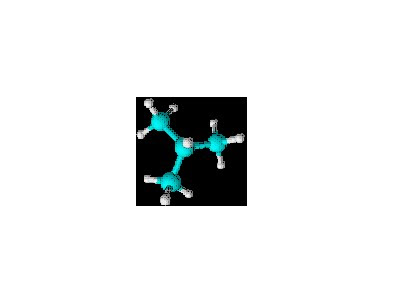
Name: isobutane (or 2-methylpropane)
Molecular formula: C4H10
Condensed structure: CH(CH3)3
In glorious 3-D:
 Both molecules have the same quantities of the same atoms. But the bonds are not identical here. A carbon bonded to two other carbons and two hydrogens is electromagnetically different from one bonded to three other carbons and one hydrogen. Also, the three-dimensional forms are quite different, and when the molecules interact with other bodies (including other molecules just like themselves) the results will be at least slightly different. Although very similar, these two compounds have different chemical and physical properties. They are more like each other than other compounds that have different atoms and other, more striking differences. Because of these facts, we use the term "constitutional isomers" to denote the relationship between these similar molecules.
Both molecules have the same quantities of the same atoms. But the bonds are not identical here. A carbon bonded to two other carbons and two hydrogens is electromagnetically different from one bonded to three other carbons and one hydrogen. Also, the three-dimensional forms are quite different, and when the molecules interact with other bodies (including other molecules just like themselves) the results will be at least slightly different. Although very similar, these two compounds have different chemical and physical properties. They are more like each other than other compounds that have different atoms and other, more striking differences. Because of these facts, we use the term "constitutional isomers" to denote the relationship between these similar molecules.
But when it comes to properties, constitutional isomers are not always so similar to each other as those two. Some constitutional isomers contain different functional groups from each other and, if you remember the importance of functional groups like you should, this means they can have dramatically different chemical and physical properties...
Name: ethanol
Molecular formula: C2H6O
Condensed structure: CH3CH2OH
In brilliant 3-D:
 It's an old friend: ethanol. I don't know how many times I've shown ethanol before, but you had better know that this is what it looks like. And if you managed to actually have some brain capacity, maybe you even remember that this compound is an alcohol, as it has a hydroxyl functional group. Easy, but here's a constitutional isomer of ethanol.
It's an old friend: ethanol. I don't know how many times I've shown ethanol before, but you had better know that this is what it looks like. And if you managed to actually have some brain capacity, maybe you even remember that this compound is an alcohol, as it has a hydroxyl functional group. Easy, but here's a constitutional isomer of ethanol.
Name: dimethyl ether (or methoxymethane)
Molecular formula: C2H6O
Condensed structure: CH3OCH3
In spectacular 3-D: Since the name has "ether" in it, you have deduced, unless you are a total idiot, that this is an ether (the name of the functional group is methoxy in this case). But the molecular formula is the same. The functional groups here are so unlike each other that reactions possible for one would be impossible for the other. Oh, and remember hydrogen bonding? Ethanol has it. Dimethyl ether cannot have hydrogen bonding because there is no hydrogen attached to the oxygen, so these two even have different intermolecular forces. In this way, two constitutional isomers can be quite dissimilar. What kind of atoms a molecule has and how many are very important, but the configuration of the bonds holding the atoms together in a molecule matters a lot too.
Since the name has "ether" in it, you have deduced, unless you are a total idiot, that this is an ether (the name of the functional group is methoxy in this case). But the molecular formula is the same. The functional groups here are so unlike each other that reactions possible for one would be impossible for the other. Oh, and remember hydrogen bonding? Ethanol has it. Dimethyl ether cannot have hydrogen bonding because there is no hydrogen attached to the oxygen, so these two even have different intermolecular forces. In this way, two constitutional isomers can be quite dissimilar. What kind of atoms a molecule has and how many are very important, but the configuration of the bonds holding the atoms together in a molecule matters a lot too.
Edit: After posting this, I started going back to tag my posts. I noticed that way back in February, I wrote a post about constitutional isomers. I think this new post is better, but here is the old one. If you do not get the concept after reading this post, read the old one. If you still don't get it, tell me, I guess. It seems fairly simple to me and I think I did an adequate job of explaining it both times, but maybe I am wrong...
Yay for 3-d!
ReplyDelete